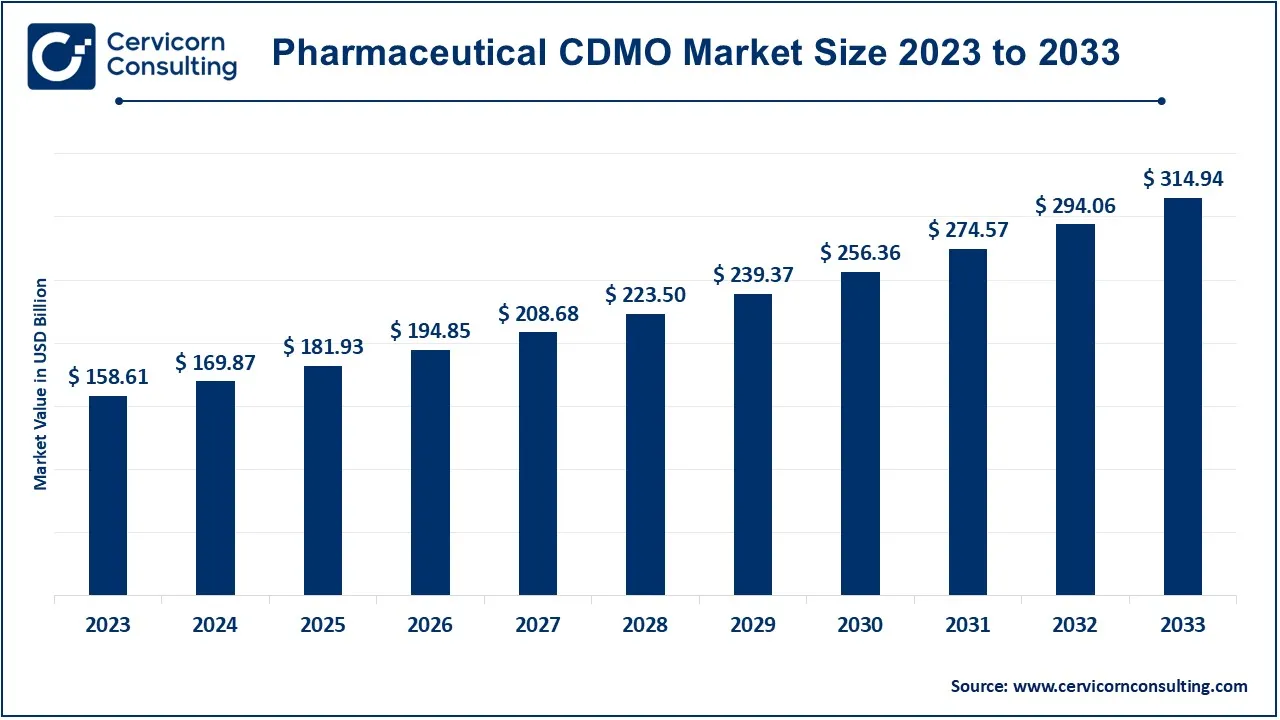
The global pharmaceutical CDMO market size was valued at USD 169.87 billion in 2024 and is expected to be worth around USD 314.94 billion by 2033. It is growing at a compound annual growth rate (CAGR) of 7.10% from 2024 to 2033.
The pharmaceutical CDMO market is expanding rapidly due to increasing outsourcing trends in the pharmaceutical and biotech sectors. Companies are turning to CDMOs to reduce production costs, shorten drug development timelines, and access advanced technologies. The rise in demand for biologics, gene therapies, and personalized medicines has created a significant need for specialized manufacturing capabilities, which CDMOs are well-equipped to provide. Additionally, as regulatory requirements grow more stringent, pharmaceutical companies rely on CDMOs for their expertise in compliance and quality assurance. Emerging markets, particularly in Asia-Pacific, are playing a crucial role in the market's expansion due to lower labor costs, growing manufacturing infrastructure, and government support for the pharmaceutical sector. In 2022, pharmaceutical products were the world's 7th most traded product, with a total trade value of USD 835 billion, representing 3.52% of total world trade. The CDMO industry also benefits from a surge in demand for innovative therapies and increased funding for research and development globally.
A Pharmaceutical CDMO (Contract Development and Manufacturing Organization) is a company that provides outsourced services for drug development and manufacturing to pharmaceutical and biotechnology companies. These organizations handle tasks such as formulating drugs, conducting clinical trials, scaling up production, and ensuring compliance with regulatory standards. By outsourcing to CDMOs, pharmaceutical companies can focus on innovation and market strategies while leveraging the expertise and infrastructure of CDMOs to reduce costs, time, and risks in drug development and manufacturing. CDMOs offer end-to-end solutions, from preclinical research to commercial production, making them essential partners in the pharmaceutical supply chain.
Report Highlights
Report Scope
| Area of Focus | Details |
| Market Size in 2024 | USD 169.87 Billion |
| Projected Market Size (2033) | USD 314.94 Billion |
| Growth Rate (2024 to 2033) | 7.10% |
| Largest Regional Share | Asia Pacific |
| Fastest Growing Region | North America |
| Segments Covered | Services, Product, Workflow, Indication, End User, Region |
| Key Players | Lonza Group AG, Catalent, Inc., Recipharm AB, Alcami Corporation, Samsung Biologics Co., Ltd., WuXi AppTec, Inc., Fujifilm Diosynth Biotechnologies, Boehringer Ingelheim GmbH, Pfizer CentreOne, Charles River Laboratories International, Inc., Evonik Industries AG, Siegfried Holding AG, Cobra Biologics, Aenova Group, NantPharma |
Increased Focus on R&D and Innovation:
Contractual Flexibility and Scalability:
Regulatory Compliance and Quality Control:
Intellectual Property Risks:
Expansion into Emerging Markets:
Advancements in Biotechnology:
Integration of Advanced Technologies
Talent Acquisition and Retention:
The pharmaceutical CDMO market is segmented into service, product, indication, end users, and region. Based on service, the market is classified into development services, manufacturing services, packaging services, and testing services. Based on product, the market is classified into small molecules, and biologics. Based on indication, the market is classified into cancer, cardiovascular disease, diabetes, pain, respiratory disease, and others. Based on end users, the market is classified into pharmaceutical companies, biotechnology companies, generic drug manufacturers, and nutraceutical companies.
Development Services: Development services in the Pharmaceutical CDMO market encompass preclinical and clinical development, formulation, analytical development, and regulatory affairs. These services support drug discovery, optimization, and regulatory compliance. There's a rising trend in personalized medicine and biologics, driving demand for advanced development services. Increasing complexity in drug formulations and regulatory requirements is leading to greater reliance on CDMOs for specialized expertise and accelerated development timelines.
Manufacturing Services: Manufacturing services include API production, finished dosage form manufacturing, packaging, and labeling. These services cover the entire production process, from synthesizing active ingredients to producing the final product. The shift towards biologics and complex molecules is expanding the scope of manufacturing services. There is also an emphasis on automation and scaling up production to meet increasing global demand, while ensuring high quality and compliance.
Packaging Services: Packaging services involve primary and secondary packaging and serialization of pharmaceutical products. These services ensure product protection, compliance with regulations, and facilitate distribution. Growing focus on patient safety and anti-counterfeiting measures is driving advancements in packaging technologies. Serialization and smart packaging are becoming more prevalent, improving traceability and security throughout the supply chain.
Testing Services: Testing services include stability, quality control, and microbiological testing. These services ensure the safety, efficacy, and quality of pharmaceutical products through rigorous testing procedures. There is an increasing demand for comprehensive testing services due to stricter regulatory standards and the need for high-quality assurance. Innovations in analytical techniques and the growth of biologics are driving advancements in testing methodologies and capacities.
Small Molecules: Small molecules are low molecular weight compounds, typically less than 900 Daltons, used in pharmaceutical drugs. They are synthesized chemically and can penetrate cells easily to exert their therapeutic effects. The market for small molecules in CDMO is growing due to increased demand for generic drugs and new small-molecule drugs. Advances in synthetic chemistry and continuous manufacturing are improving efficiency and reducing costs.
Biologics: Biologics are large, complex molecules or mixtures of molecules derived from living organisms. They include products like monoclonal antibodies, vaccines, and cell and gene therapies. The biologics sector is rapidly expanding, driven by advancements in biotechnology and personalized medicine. CDMOs are investing in advanced facilities and technologies to handle the complex production processes of biologics, including cell culture and fermentation. Increased focus on precision medicine and immunotherapies is further boosting growth in this segment.
Cancer: Cancer treatments often involve complex drugs, including biologics and small molecules. CDMOs provide development and manufacturing services for oncology drugs, ensuring high quality and compliance with regulatory standards. Increasing investment in cancer research has driven demand for specialized CDMO services. There's a growing emphasis on personalized medicine and advanced therapies like CAR-T cells, necessitating sophisticated manufacturing capabilities.
Cardiovascular Disease: CDMOs support the development of drugs and biologics for cardiovascular diseases, including hypertension, heart failure, and atherosclerosis. These services cover the entire spectrum from preclinical to commercial production. There is a rising focus on novel therapies for cardiovascular diseases. Advanced drug delivery systems and biologics are gaining traction, leading to increased demand for CDMO expertise in these areas.
Diabetes: The management of diabetes involves insulin and other drug formulations. CDMOs offer services to develop, manufacture, and package these critical diabetes treatments. The demand for diabetes treatments is growing due to rising prevalence. Innovations like insulin pumps and continuous glucose monitors are driving the need for advanced manufacturing solutions and new drug formulations.
Pain: CDMOs provide services for developing and manufacturing analgesics and other pain management therapies. This includes both over-the-counter and prescription medications. There is a shift towards developing non-opioid pain management solutions due to the opioid crisis. CDMOs are focusing on novel drug delivery systems and biologics to meet this evolving need.
Respiratory Disease: For respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD), CDMOs develop and manufacture inhaled therapies and biologics. The rise in respiratory conditions and demand for innovative inhalers and biologics are boosting the CDMO market. Advances in targeted therapies and personalized medicine are further driving growth in this sector.
Others: This category includes CDMO services for a variety of other conditions, such as neurological disorders, autoimmune diseases, and infectious diseases. The expansion into rare and niche diseases is increasing. CDMOs are adapting to the growing need for specialized treatments and orphan drugs, with a focus on tailored solutions and advanced technologies.
Pharmaceutical Companies: Pharmaceutical companies, leveraging CDMO services, focus on outsourcing drug development and manufacturing to reduce costs and expedite time-to-market. Trends include increased demand for specialized services, like advanced formulation and high-potency API manufacturing, driven by the need for complex drug development and regulatory compliance.
Biotechnology Companies: Biotechnology companies utilize CDMOs for their expertise in biologics and complex therapeutic products. Trends show a rising need for CDMOs with capabilities in biologics production, including monoclonal antibodies and cell therapies, as biotech firms focus on innovative treatments and personalized medicine.
Generic Drug Manufacturers: Generic drug manufacturers turn to CDMOs for cost-effective and scalable production solutions. Trends include a shift towards outsourcing to manage production costs and meet stringent regulatory requirements, while also focusing on speed to market for generic drugs amidst increasing competition.
Nutraceutical Companies: Nutraceutical companies collaborate with CDMOs to develop and manufacture dietary supplements and functional foods. Trends indicate a growing demand for high-quality, scientifically validated products and advanced packaging solutions, driven by consumer health awareness and regulatory scrutiny.
Others: This category encompasses various end users such as medical device companies and contract research organizations (CROs). Trends involve increased outsourcing to CDMOs for specialized manufacturing and development needs, reflecting a broader adoption of contract services across the healthcare and wellness sectors.
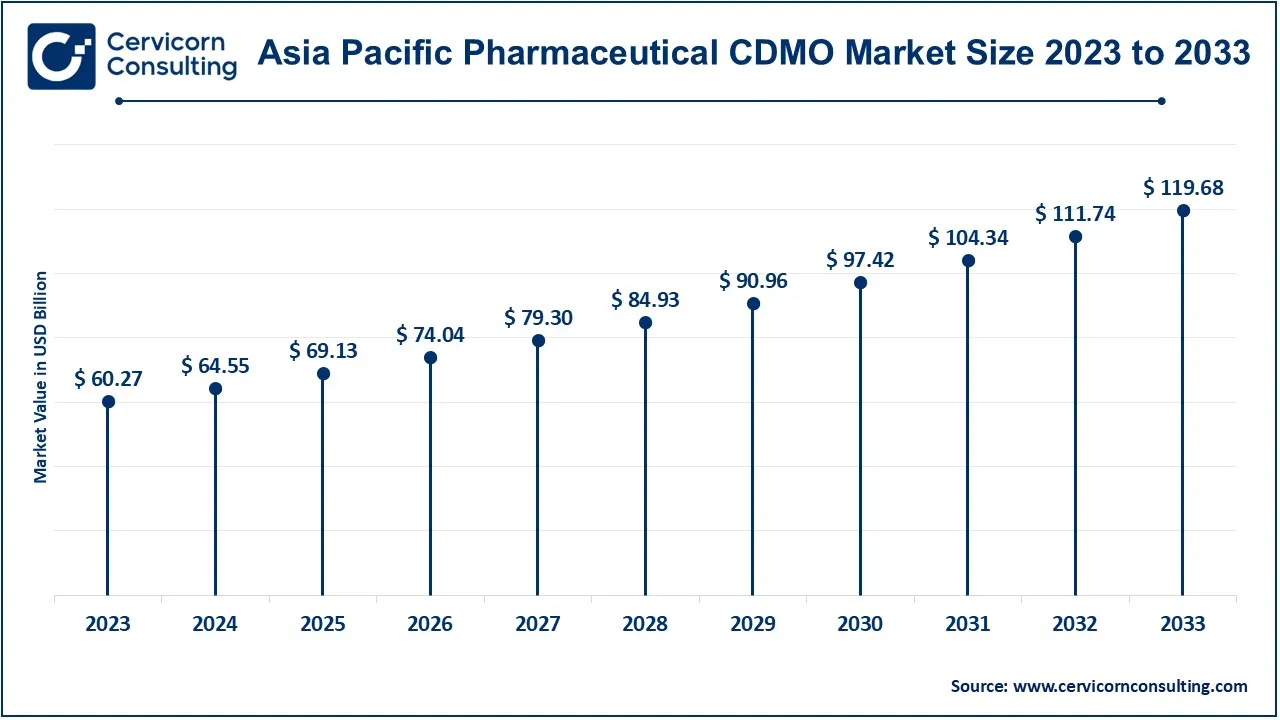
The Asia-Pacific pharmaceutical CDMO market size woth at USD 64.55 billion in 2024 and is expected to reach around USD 119.68 billion by 2033. The Asia-Pacific region is leading, due to its expanding pharmaceutical and biotechnology industries. Trends include a surge in outsourcing activities driven by cost advantages, increasing investments in state-of-the-art facilities, and rising demand for high-quality production services to cater to both local and global markets.
The North America pharmaceutical CDMO market size accounted for USD 40.77 billion in 2024 and is projected to hit around USD 75.59 billion by 2033. In North America is driven by a high concentration of pharmaceutical and biotech companies, leading to strong demand for contract manufacturing services. Trends include a growing emphasis on advanced manufacturing technologies and regulatory compliance, as well as increasing partnerships and mergers among CDMOs to expand capabilities and geographical reach.
The Europe pharmaceutical CDMO market size is estimated at USD 52.66 billion in 2024 and is anticipated to surpass around USD 97.63 billion by 2033. The Europe market is characterized by a focus on innovation and regulatory adherence, with a significant demand for CDMOs specializing in biologics and complex generics. Trends include increased investment in cutting-edge manufacturing technologies and a rise in cross-border collaborations due to stringent European regulatory standards and diverse pharmaceutical needs.
The LAMEA pharmaceutical CDMO market size is calculated at USD 11.89 billion in 2024 and is projected to reach around USD 22.05 billion by 2033. The LAMEA market is growing due to rising healthcare infrastructure and pharmaceutical production capacities. Trends include increased outsourcing by local pharmaceutical companies seeking cost efficiencies, a focus on expanding capabilities in emerging markets, and collaborations aimed at meeting the region's diverse regulatory requirements and market needs.
Recent entrants like Aenova Group and Cobra Biologics have adopted advanced technologies such as continuous manufacturing and cell and gene therapy to differentiate themselves in the pharmaceutical CDMO market. They are investing in state-of-the-art facilities and specialized services to cater to evolving client needs. Lonza Group AG and Catalent, Inc. are market leaders due to their extensive global presence and comprehensive service offerings. Lonza excels in biologics and small molecules, while Catalent's expertise spans across clinical and commercial supply, driving their market dominance.
The pharmaceutical CDMO market has seen several key developments in recent years, with companies seeking to expand their market presence and leverage synergies to improve their offerings and profitability. Some notable examples of key development in the market include:
This key development helped companies expand their offerings, improve their market presence, and capitalize on growth opportunities in the pharmaceutical CDMO Market. The trend is expected to continue as companies seek to gain a competitive edge in the market.
Market Segmentation
By Services
By Product
By Indication
By Workflow
By End User
By Regions
Chapter 1 Market Introduction and Overview
1.1 Market Definition and Scope
1.1.1 Overview of Pharmaceutical CDMO
1.1.2 Scope of the Study
1.1.3 Research Timeframe
1.2 Research Methodology and Approach
1.2.1 Methodology Overview
1.2.2 Data Sources and Validation
1.2.3 Key Assumptions and Limitations
Chapter 2 Executive Summary
2.1 Market Highlights and Snapshot
2.2 Key Insights by Segments
2.2.1 By Services Overview
2.2.2 By Product Overview
2.2.3 By Indication Overview
2.2.4 By Workflow Overview
2.2.5 By End User Overview
2.3 Competitive Overview
Chapter 3 Global Impact Analysis
3.1 COVID 19 Impact on Pharmaceutical CDMO Market
3.1.1 COVID-19 Landscape: Pre and Post COVID Analysis
3.1.2 COVID 19 Impact: Global Major Government Policy
3.1.3 Market Trends and Opportunities in the COVID-19 Landscape
3.2 Russia-Ukraine Conflict: Global Market Implications
3.3 Regulatory and Policy Changes Impacting Global Markets
Chapter 4 Market Dynamics and Trends
4.1 Market Dynamics
4.1.1 Market Drivers
4.1.1.1 Increased Focus on R&D and Innovation
4.1.1.2 Contractual Flexibility and Scalability
4.1.2 Market Restraints
4.1.2.1 Regulatory Compliance and Quality Control
4.1.2.2 Intellectual Property Risks
4.1.3 Market Opportunity
4.1.3.1 Expansion into Emerging Markets
4.1.3.2 Advancements in Biotechnology
4.1.4 Market Challenges
4.1.4.1 Integration of Advanced Technologies
4.1.4.2 Talent Acquisition and Retention
4.2 Market Trends
Chapter 5 Premium Insights and Analysis
5.1 Global Pharmaceutical CDMO Market Dynamics, Impact Analysis
5.2 Porter’s Five Forces Analysis
5.2.1 Bargaining Power of Suppliers
5.2.2 Bargaining Power of Buyers
5.2.3 Threat of Substitute Products
5.2.4 Rivalry among Existing Firms
5.2.5 Threat of New Entrants
5.3 PESTEL Analysis
5.4 Value Chain Analysis
5.5 Product Pricing Analysis
5.6 Vendor Landscape
5.6.1 List of Buyers
5.6.2 List of Suppliers
Chapter 6 Pharmaceutical CDMO Market, By Services
6.1 Global Pharmaceutical CDMO Market Snapshot, By Services
6.1.1 Market Revenue (($Billion) and Growth Rate (%), 2021-2033
6.1.1.1 Development Services
6.1.1.2 Manufacturing Services
6.1.1.3 Packaging Services
6.1.1.4 Testing Services
Chapter 7 Pharmaceutical CDMO Market, By Product
7.1 Global Pharmaceutical CDMO Market Snapshot, By Product
7.1.1 Market Revenue (($Billion) and Growth Rate (%), 2021-2033
7.1.1.1 Small Molecules
7.1.1.2 Biologics
Chapter 8 Pharmaceutical CDMO Market, By Indication
8.1 Global Pharmaceutical CDMO Market Snapshot, By Indication
8.1.1 Market Revenue (($Billion) and Growth Rate (%), 2021-2033
8.1.1.1 Cancer
8.1.1.2 Cardiovascular Disease
8.1.1.3 Diabetes
8.1.1.4 Pain
8.1.1.5 Respiratory Disease
8.1.1.6 Infectious Diseases
8.1.1.7 Neurological Disorders
8.1.1.8 Metabolic Disorders
8.1.1.9 Autoimmune Diseases
8.1.1.10 Ophthalmology
8.1.1.11 Gastrointestinal Disorders
8.1.1.12 Hormonal Disorders
8.1.1.13 Hematological Disorders
8.1.1.14 Others
Chapter 9 Pharmaceutical CDMO Market, By Workflow
9.1 Global Pharmaceutical CDMO Market Snapshot, By Workflow
9.1.1 Market Revenue (($Billion) and Growth Rate (%), 2021-2033
9.1.1.1 Commercial
9.1.1.2 Clinical
Chapter 10 Pharmaceutical CDMO Market, By End User
10.1 Global Pharmaceutical CDMO Market Snapshot, By End User
10.1.1 Market Revenue (($Billion) and Growth Rate (%), 2021-2033
10.1.1.1 Pharmaceutical Companies
10.1.1.2 Biotechnology Companies
10.1.1.3 Generic Drug Manufacturers
10.1.1.4 Nutraceutical Companies
10.1.1.5 Others
Chapter 11 Pharmaceutical CDMO Market, By Region
11.1 Overview
11.2 Pharmaceutical CDMO Market Revenue Share, By Region 2023 (%)
11.3 Global Pharmaceutical CDMO Market, By Region
11.3.1 Market Size and Forecast
11.4 North America
11.4.1 North America Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.4.2 Market Size and Forecast
11.4.3 North America Pharmaceutical CDMO Market, By Country
11.4.4 U.S.
11.4.4.1 U.S. Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.4.4.2 Market Size and Forecast
11.4.4.3 U.S. Market Segmental Analysis
11.4.5 Canada
11.4.5.1 Canada Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.4.5.2 Market Size and Forecast
11.4.5.3 Canada Market Segmental Analysis
11.4.6 Mexico
11.4.6.1 Mexico Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.4.6.2 Market Size and Forecast
11.4.6.3 Mexico Market Segmental Analysis
11.5 Europe
11.5.1 Europe Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.5.2 Market Size and Forecast
11.5.3 Europe Pharmaceutical CDMO Market, By Country
11.5.4 UK
11.5.4.1 UK Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.5.4.2 Market Size and Forecast
11.5.4.3 UK Market Segmental Analysis
11.5.5 France
11.5.5.1 France Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.5.5.2 Market Size and Forecast
11.5.5.3 France Market Segmental Analysis
11.5.6 Germany
11.5.6.1 Germany Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.5.6.2 Market Size and Forecast
11.5.6.3 Germany Market Segmental Analysis
11.5.7 Rest of Europe
11.5.7.1 Rest of Europe Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.5.7.2 Market Size and Forecast
11.5.7.3 Rest of Europe Market Segmental Analysis
11.6 Asia Pacific
11.6.1 Asia Pacific Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.2 Market Size and Forecast
11.6.3 Asia Pacific Pharmaceutical CDMO Market, By Country
11.6.4 China
11.6.4.1 China Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.4.2 Market Size and Forecast
11.6.4.3 China Market Segmental Analysis
11.6.5 Japan
11.6.5.1 Japan Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.5.2 Market Size and Forecast
11.6.5.3 Japan Market Segmental Analysis
11.6.6 India
11.6.6.1 India Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.6.2 Market Size and Forecast
11.6.6.3 India Market Segmental Analysis
11.6.7 Australia
11.6.7.1 Australia Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.7.2 Market Size and Forecast
11.6.7.3 Australia Market Segmental Analysis
11.6.8 Rest of Asia Pacific
11.6.8.1 Rest of Asia Pacific Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.6.8.2 Market Size and Forecast
11.6.8.3 Rest of Asia Pacific Market Segmental Analysis
11.7 LAMEA
11.7.1 LAMEA Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.7.2 Market Size and Forecast
11.7.3 LAMEA Pharmaceutical CDMO Market, By Country
11.7.4 GCC
11.7.4.1 GCC Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.7.4.2 Market Size and Forecast
11.7.4.3 GCC Market Segmental Analysis
11.7.5 Africa
11.7.5.1 Africa Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.7.5.2 Market Size and Forecast
11.7.5.3 Africa Market Segmental Analysis
11.7.6 Brazil
11.7.6.1 Brazil Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.7.6.2 Market Size and Forecast
11.7.6.3 Brazil Market Segmental Analysis
11.7.7 Rest of LAMEA
11.7.7.1 Rest of LAMEA Pharmaceutical CDMO Market Revenue, 2021-2033 ($Billion)
11.7.7.2 Market Size and Forecast
11.7.7.3 Rest of LAMEA Market Segmental Analysis
Chapter 12 Competitive Landscape
12.1 Competitor Strategic Analysis
12.1.1 Top Player Positioning/Market Share Analysis
12.1.2 Top Winning Strategies, By Company, 2021-2023
12.1.3 Competitive Analysis By Revenue, 2021-2023
12.2 Recent Developments by the Market Contributors (2023)
Chapter 13 Company Profiles
13.1 Lonza Group AG
13.1.1 Company Snapshot
13.1.2 Company and Business Overview
13.1.3 Financial KPIs
13.1.4 Product/Service Portfolio
13.1.5 Strategic Growth
13.1.6 Global Footprints
13.1.7 Recent Development
13.1.8 SWOT Analysis
13.2 Catalent, Inc.
13.3 Recipharm AB
13.4 Alcami Corporation
13.5 Samsung Biologics Co., Ltd.
13.6 WuXi AppTec, Inc.
13.7 Fujifilm Diosynth Biotechnologies
13.8 Boehringer Ingelheim GmbH
13.9 Pfizer CentreOne
13.10 Charles River Laboratories International, Inc.