U.S. Digital Biomarkers Market Size and Growth 2025 to 2034
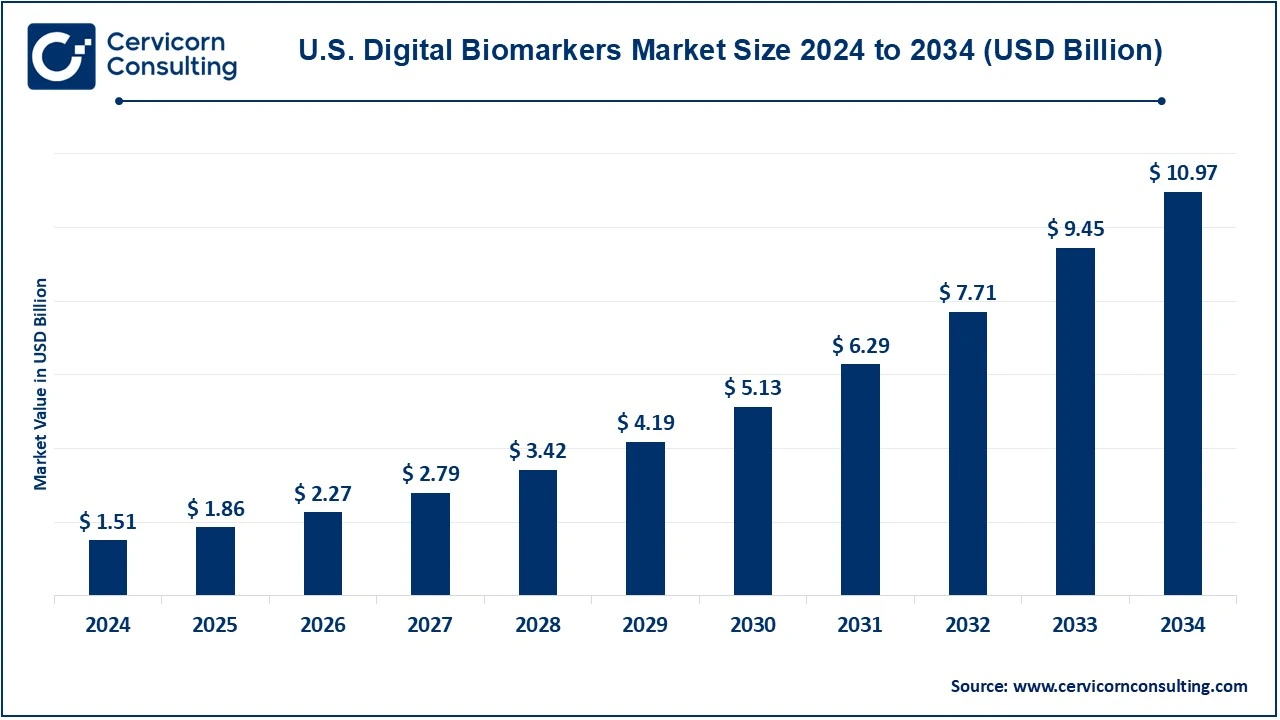
The U.S. digital biomarkers market size was valued at USD 1.51 billion in 2024 and is expected to reach around USD 10.97 billion by 2034, growing at a compound annual growth rate (CAGR) of 21.93% over the forecast period 2025 to 2034.
The growth in the market is mainly driven by technological advancements, mainly wearables, mobile health applications, and sensors that make continuous health data monitoring feasible at the point of time in a real-time manner. They make for more accurate and personal information about an individual's health enable better control of chronic diseases and can anticipate illnesses in advance. Additionally, factors like increased demand for remote patient monitoring, lifestyle-related diseases' growing prevalence, and value-based healthcare models are changing the scene. The clinical trials become more efficient, thus expediting the new treatment's development, due to digital biomarkers.
CEO Statements
Padraig McDonnell, CEO of Agilent Technologies, Inc.
- "As I think about Agilent's future, I am excited by the endless possibilities and opportunities that lie in front of us as we help our customers bring great science to life"
Norman Schwartz, CEO of Bio-Rad Laboratories, Inc.
- "We recognize the importance of digital biomarkers in advancing healthcare and diagnostics. Our commitment is to leverage our extensive technology platform and expertise to innovate in this space, ensuring that we provide valuable solutions that meet the evolving needs of our customers."
Dirk Bontridder, CEO of PerkinElmer Inc.:
- "The fundamentals of the new PerkinElmer business are strong. We are committed to leveraging our innovative portfolio to enhance scientific outcomes in healthcare. Digital biomarkers play a crucial role in this transformation, and we aim to develop solutions that not only meet but exceed the evolving needs of our customers."
U.S. Digital Biomarkers Market Growth Factors
- Increased investment in healthcare technology: This has been driven by the development of digital biomarkers by strong funding coming from venture capital and government initiatives. Increasing interest in healthcare innovation is further increasing investor interest in backing new firms and established firms developing wearable and sensor technologies. The ecosystem encourages rapid growth and market expansion.
- Increased Demand for Personalized Medicine: Mainly because this move toward precision medicine customization of the treatment plans based on specific genetic and lifestyle relies mainly on digital biomarkers-it will allow real-time monitoring of the health condition and, therefore, enable the design of better-targeted treatments with better efficacy and outcome, which in turn shall give a boost to market growth.
- Integration with Telemedicine: A fast-emerging trend in medicine that has brought along the perfect opportunity for digital biomarkers is telemedicine. Since patients can be monitored via remote patient monitoring, thus digital biomarkers can use such data to improve health in terms of making less personal visit diagnoses and treatments for healthcare providers.
U.S. Digital Biomarkers Market Trends
- Increased Use of AI and Machine Learning: In a digital biomarker platform, artificial intelligence, and machine learning are integrated to analyze massive amounts of data to recognize various patterns. The precision of diagnosis is improved along with the speed of diagnosis because healthcare professionals can now easily detect diseases earlier and in more precise ways, therefore propelling the market further.
- Mobile health (mHealth) applications and devices: These following digital biomarkers are increasingly becoming popular because they are accessible and convenient to use. They empower patients to track their conditions continuously, giving them and healthcare providers real-time data that can be used to manage situations and prevent health issues from getting worse.
- Tech and Health Companies Collaborations: Collaboration between the technology companies and healthcare providers for innovation in the digital biomarker solution is now at a peak for integration of better health data, user-friendly devices, and products that can easily find their place in clinical settings.
Report Scope
| Are of Focus |
Details |
| Market Size in 2025 |
USD 1.86 Billion |
| Expected Market Size in 2034 |
USD 10.97 Billion |
| CAGR |
21.93% |
| Key Segments |
Type, Disease, Clinical Practice, End-use |
| Key Companies |
Bio-Rad Laboratories, Inc., Abbott, Agilent Technologies, Inc., Johnson & Johnson Services, Inc., Thermo Fisher Scientific, Inc., PerkinElmer Inc., General Electric, MESO SCALE DIAGNOSTICS |
U.S. Digital Biomarkers Market Dynamics
Drivers
- Increasing Adoption of Personalized Medicine: This is the reason personalized medicine by the medical industry necessitates an increasing rate of digital biomarkers. The applications allow health workers to give more accurate treatments, which means better patient care and stronger efficacy of therapies and thus quicker take-up in various fields of medicine for digital biomarkers.
- Regulatory Approaches for Digital Health Solutions: Regulators, such as the FDA, have started taking in digital health devices and applications as proof of the devices' reliability and safety. Validation increases market confidence; thus, more health providers will accept the utilization of digital biomarkers hence creating the market by adhering to regulatory standards.
- Cost-Efficiency in Healthcare: Digital biomarkers are cost-effective ways to monitor chronic conditions and enhance disease management. The ability of digital biomarkers to reduce hospital visits and enable early intervention leads to a substantial reduction in healthcare costs. The increasing demand for cost-effective healthcare solutions is, therefore, an important factor in the expansion of the market.
Restraints
- Lack of knowledge among healthcare providers: Many healthcare professionals are not familiar with digital biomarkers and their potential benefits. Unless health care professionals receive proper training and education on these new tools, adoption in clinical practice will be slower, which will deter the widespread use of digital biomarkers in mainstream health care.
- Lack of Fragmentation in Market: The market is a highly fragmented with multiple players that propose different technologies without interoperability standards. This could, in principle, create some difficulties to patients and health providers trying to integrate their solutions within multiple platforms and hence these technologies are limited in scaling.
- Complexity of Data Interpretation: The sheer volume of data generated by digital biomarkers makes interpretation a very complex task requiring sophisticated algorithms and expertise. Without appropriate data analysis tools, healthcare providers will find it challenging to make full use of digital biomarkers, which will be their major drawback in clinical applications.
Opportunities
- AI & Machine Learning Integration: There is a large opportunity in this direction, and it's interesting as AI/ML algorithms may analyze such huge datasets provided by the digital biomarkers to understand the subtlest of the patterns and identify health events even before the event happens. With this approach, healthcare professionals can act in advance on such preventive interventions. At the same time, with these technologies, diagnostics improves in accuracy so that proper treatment becomes better targeted as well. The advancement in AI and ML leads to the predictive analytics abilities of healthcare with greater accuracy for improved patient outcomes.
- Healthcare Reaching Remote Rural Areas: The application of digital biomarkers promises to improve the accessibility of health care considerably, especially to people residing in rural or other under-served areas. Accessing special care facilities often presents geographic and monetary challenges that hinder traditional systems of healthcare. Digital biomarkers coupled with telemedicine and remote monitoring technologies allow the continuity of health monitoring outside of clinical settings and thereby overcome the gap left by such limitations. This remote monitoring capability will allow patients in rural settings to receive timely interventions and personalized care that ensure health outcomes without the need to travel frequently.
- Better Patient Engagement: Enhanced through digital biomarkers, increased patients' engagement through real-time observations of their health status through wearables and mobile applications can allow the patient him/herself to collect their own health metrics information. Such information fosters more self-awareness of healthy behavior and compels them towards better practices. As such, monitoring continues, empowering the understanding of the cause-and-effect relationship of his/her doings for being healthy or not. For patients, the digital biomarkers increase accountability to facilitate better long-term health outcomes and quality of life.
Challenges
- Cost of Development: The development of digital biomarkers is a financially expensive investment, especially for small companies and startups. Research and technology development and clinical trials are expensive activities requiring resources, time, and expertise. Ongoing maintenance, data infrastructure, and software development all add to the financial costs. For many companies, getting funding for such high-risk, high-cost projects will be a significant barrier. These financial constraints make the innovations slower, limit entry by the smaller players, and delay the spread of digital biomarkers into routine clinical practice.
- Clinical Integration: The integration of such digital biomarkers into pre-existing clinical workflows is another complex challenge. Healthcare systems as well as medical practices that are traditionally designed upon conventionally used diagnostic tools and person-to-person patient visits are not supportive of continuous surveillance from a distance. There is a great challenge in adapting data management, medical guidelines, and healthcare staff pieces of training. In addition to this, the integration of digital biomarkers into an electronic health record and various other clinical systems requires consensus among multiple stakeholders. However, the integration of diverse stakeholders is crucial for easy integration into healthcare delivery systems.
- Technical Challenges: It's probably the biggest limitation that faces the adoption of digital biomarkers: technical limitation. Most digital devices and sensors lack accuracy, reliability, and consistency. Wearables may not accurately record health data at all times; environmental factors like movement and temperature may affect readings. Moreover, most digital biomarkers rely on constant data streams, which can be susceptible to battery life, connectivity problems, and technical malfunctions. Such limitations can defeat the purposes of digital biomarkers and may become a risk to the safety of patients, hence cannot be embraced and applied in clinical environments.
U.S. Digital Biomarkers Market Segmental Analysis
The U.S. digital biomarkers market is segmented into type, disease, clinical practice and end user. Based on type, the market is classified into validation, efficacy, and safety. Based on disease, the market is classified into neurological diseases, cancer, immunological diseases, respiratory diseases, musculoskeletal diseases, cardiovascular diseases, and others. Based on clinical practice, the market is classified into personalized medicines, drug discovery & development, and diagnostics. Based on end-use, the market is classified into healthcare companies, healthcare providers, payers, and others.
Type Analysis
Validation: To be validated, the digital biomarkers have to ensure the reflection of data produced by devices or sensors in digital settings is precise, representing the state of biology they are purported to measure. This is fundamental to ensuring that the biomarkers generated are reliable and clinically applicable. Extensive validation studies are required for approval by regulatory bodies such as the FDA for the use of these digital biomarkers in clinical settings. The use of such biomarkers without validation can be prone to misdiagnosis and ineffective treatment, thereby lowering the utility of these biomarkers in medicine.
Efficiency: Efficacy here refers to the ability of digital biomarkers to predict or monitor the effects of a medical intervention or progression of a disease. Because clinical practitioners have real-time data, they can assess the efficacy of treatment and even change treatment plans.It is possible with real-time tracking of health metrics, which allows insight into whether treatments are indeed optimized, and patients receive positive responses. The clinical adoption and regulatory approval of digital biomarkers in healthcare practices require demonstration of efficacy.
Safety: Safety is the top issue when digital biomarkers are used in healthcare because inaccurate readings or faulty devices can expose the patient to harm. In ensuring the success of clinical use, it is crucial that the digital biomarkers do not, either by false readings or failing of devices. The safety issues are primarily overcome through stringent testing, regulatory oversight, and constant monitoring of the digital devices so that they function in accordance with the stipulated safety standards. The long-term safety of the digital biomarkers should have no harmful influence on the patients' physical and psychological conditions.
Diseases Analysis
Neurological Diseases: With increasing care and monitoring in cases of neurological diseases-from Alzheimer's to Parkinson's, and multiple sclerosis-they now have more of a need for digital biomarkers. All these diseases require constant observation for knowing the progress the disease is making or whether the patient is responding positively to treatment. It follows that digital biomarkers - through activity trackers and cognitive tests of the patient - are an important tool for monitoring and possibly improving motor functions, the level of cognitive abilities and behavioral patterns. It thus also allows for better calibration of treatment and provides qualitative improvements to the lives of neurologic disease patients by delivering an immediate view into a patient's state of health.
Cancer: The digital biomarkers for approaching early detection, management of therapy, and surveillance of growth of the tumor in cancer would be any physiological changes pointing toward molecular markers associated with growth of the tumor or response and potential metastasis. The digital tools are those like wearables and diagnostic apps that can detect even slight changes in patient health reflecting a change in status of the cancer. Continuous monitoring by digital biomarkers allow clinicians to catch recurrences early, better tailor treatments, and improve patient outcomes in the fight against cancer.
Immunological Disorders: Immunological diseases that are categorized under autoimmune and chronic inflammatory conditions benefit due to digital biomarkers since they are able to measure the activities of the immune system as well as exacerbations. Digital biomarkers can be monitored in a real-time basis with devices such as wearable devices on physical metrics or sensors assessing the cytokine level in patients' bodies.This stream of continuous data enables tracking of the progress of diseases, individualized treatment plans, and forecasting flare-ups for patients suffering from immunological diseases.
Cardiovascular Diseases: Digital biomarkers that monitor the below-mentioned vital parameters, such as heart rate, blood pressure, and oxygen levels, are helpful in cardiovascular diseases like heart diseases, hypertension, and arrhythmias. Wearable devices with sensors are capable of continuous and noninvasive monitoring of cardiovascular health by providing early warnings regarding such potential heart-related problems.Digital biomarkers provide timely interventions for health professionals, adjustments in medication regimens, and improved outcomes of long-term heart health. Feedback from cardiovascular biomarkers also helps in averting frequent hospital visits or emergency cases, thus enhancing the quality of life in patients.
Clinical Practice Analysis
Personalized medicines: Tailor-made medicine is a quickly evolving area, and biomarkers are the digital solutions in the process of personalized treatments for patients. Thus, with real-time data regarding a patient's disease status, digital biomarkers enable health care providers to design specific therapies based on patterns of health or genomic profiles. This ensures effective management of treatment with minimal adverse side effects. The use of digital biomarkers monitors the response of patients to medication and optimizes dosing along with progressing the disease process such that optimal treatment plans can be formulated, based on the requirement of each individual and health improvement.
Drug Discovery & Development: Digital biomarkers are one of the important players in drug discovery and development that help identify potential therapeutic targets, assess drug efficacy, and monitor adverse effects. Digital biomarkers will thus offer researchers very precise, real-time data for a deeper understanding of biological processes involved in disease conditions. They make clinical trials easier to conduct by virtue of increased accuracy in recruiting patients and tracking responses to treatments. Digital biomarkers further allow for the monitoring of biomarkers in early-stage clinical trials and thus provide more reliable data in making decisions regarding drug development.
Diagnostics: Diagnostics have totally altered the scenario of diagnosis as it presents non-invasive, constant monitoring through which diseases would be found out in a very initial phase of life. A lot of movement and related signs along with other types of physiological signals are nowadays measured by means of wearable gadgets and mobile apps that signify the concept of digital biomarkers. Biomarkers help clinicians detect anomalies that manifest before the symptoms become clinically apparent, hence earlier interventions are brought and result in better patient outcomes. Digital diagnostics on biomarkers are also cheaper than traditional diagnostic tools, making healthcare much more accessible.
U.S. Digital Biomarkers Market Top Companies
The new entrants in the digital biomarkers sector are using sophisticated technologies, such as AI, machine learning, and real-time data analytics, to improve precision and efficiency in patient monitoring and disease management. The companies are making use of wearable devices, mobile health apps, and remote sensing technologies to capture continuous real-time health data, analyze to identify patterns, predict disease progression, and customize treatments. This drives many to focus on collaboration with healthcare providers and pharmaceutical companies to integrate digital biomarkers into clinical trials, enable quicker drug development, and improve the outcomes of patients. These innovations are what are making the market grow and change rapidly and position digital biomarkers as the leading tool in precision medicine.
Recent Developments
- In September 2024, Agilent Technologies opened a new lab dedicated to Biopharma Companion Diagnostics (CDx) Services in order to accelerate precision medicine. The center will help the biopharmaceutical companies in developing and validating companion diagnostics so that it optimizes the patient's choices for treatment. Advanced technology along with expertise are used here for speeding up the whole process to make therapies address the precise needs of an individual patient. This initiative represents improvement in personalized healthcare solutions and patient outcomes concerning innovative services of biopharma diagnostics at Agilent.
- In July 2023, Bio-Rad Laboratories focused on the benefits of droplet digital PCR technology to improve the biopharma and translational research industries. Precision and sensitivity in the measurement of nucleic acids were focused on improvement by the company, given the significance of such measurements for various applications, including the discovery of biomarkers and the development of therapeutics. The ultimate goal of Bio-Rad was to offer new solutions that help researchers discover more effective diagnostics and treatments to drive progress in personalized medicine.
- In May 2024, the FDA approved a new blood test from the U.S. Army Medical Materiel Development Activity in collaboration with Abbott Point of Care for traumatic brain injury that detects faster than any other available in the past. With this i-STAT Alinity device, the biomarkers related to the head injury can be detected at raised levels within 15 minutes and results can be generated. This advancement allows for quicker medical responses in combat situations, facilitating timely transport for further evaluation. The technology incorporates biomarkers from UF startup Banyan Biomarkers, previously approved in 2018.
Market Segmentation
By Type
- Validation
- Efficacy
- Safety
By Disease
- Neurological Diseases
- Cancer
- Immunological Diseases
- Respiratory Diseases
- Musculoskeletal Diseases
- Cardiovascular Diseases
- Others
By Clinical Practice
- Personalized Medicines
- Drug Discovery & Development
- Diagnostics
By End-use
- Healthcare companies
- Healthcare Providers
- Payers
- Others
...
...
