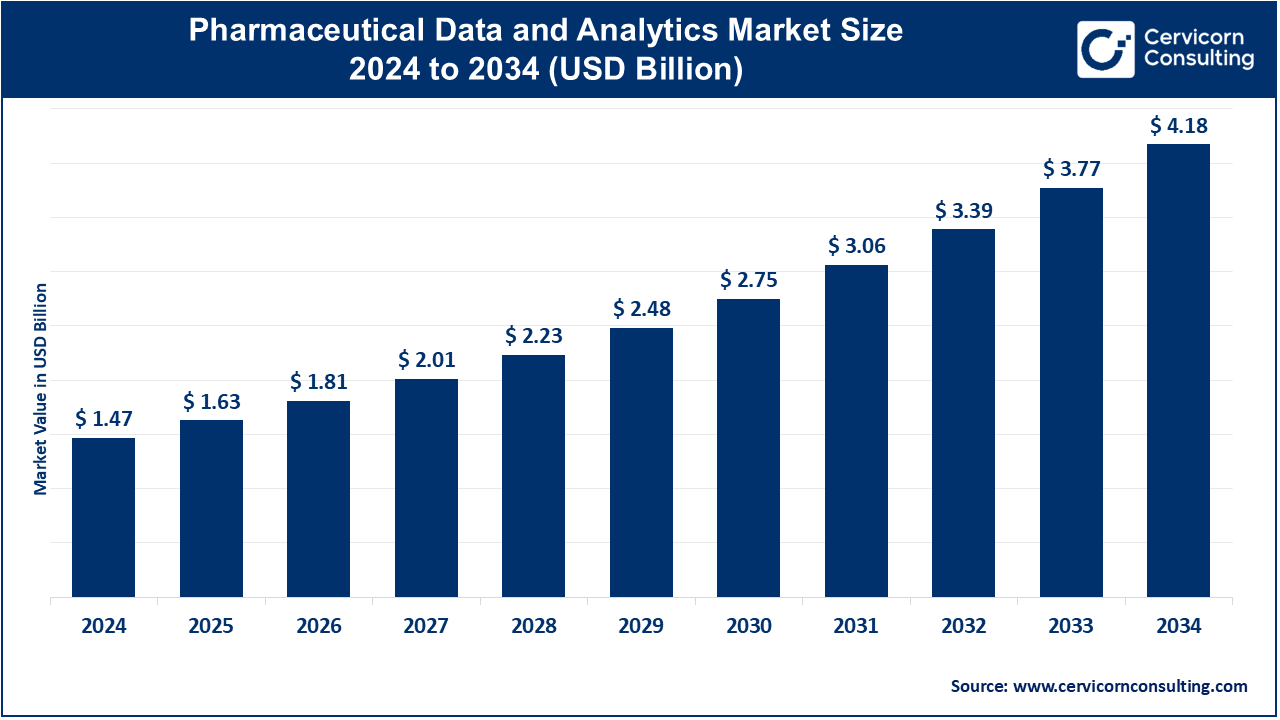
The global pharmaceutical data and analytics market size was valued at USD 1.47 billion in 2024 and is estimated to reach around USD 4.18 billion by 2034, growing at a CAGR of 11.01% during the forecast period 2025 to 2034. The pharmaceutical data and analytics market is expected to witness substantial growth owing to increasing usage of the analytical technologies for streamlining the business processes. It also has an important role in the improvement of drug discovery, optimization of clinical trials, and improving patient outcomes with the help of data-driven insights.
The pharmaceutical data and analytics market is expected to grow owing to rising demand for the data-driven decision making for development of drugs along with clinical trials and market access strategies. In the pharmaceutical sector, from widespread digital teratology testing, companies are more substantial increases in Data Mining and the advance of Artificial Intelligence (AI) and Machine Learning (ML). This has led to genuinely transformative changes in the pharmaceutical sector in terms of knowing about the strain, personalized curing, and being able to predict adverse-event likelihood while work is in progress in a trial. For instance, in the next ten years, the R&D pipeline involved serving patients, ensuring greater satisfaction, and improving disaster recovery using customized medications from a small set of cladistic similarities within commercial data sets. The surge in electronic medical records (EMRs), demand for data transparency as part of regulatory requirements, and the use of the best cloud technology have created a very satisfactory market as part of the digitization process. However, the best sales for this market are found in North America, which is because great pharmaceutical companies are sitting with strong investment in R&D and sophisticated healthcare IT facilities.
Report Scope
| Area of Focus | Details |
| Market Size in 2025 | USD 1.47 Billion |
| Projected Market Size in 2034 | USD 4.18 Billion |
| Expected CAGR from 2025 to 2034 | 11.01% |
| Dominant Area | North America |
| Leading Growth Region | Asia-Pacific |
| Key Segments | Component, Deployment Model, End User, Region |
| Key Companies | TRINITY PHARMA SOLUTIONS, CitiusTech Inc., IBM, International Business Machines Corporation, Northwest Analytics Inc., ORACLE, SCIO HEALTH ANALYTICS, Statistical Analysis System, TAKE Solutions Ltd., Wipro |
IoMT Devices
Emerging Markets Growth
Data Privacy and Security Concerns
High Implementation Costs
Integrated Analytics Platforms
Expansion in Emerging Markets
Lack of Standardization
Resistance to Technology Adoption
The pharmaceutical data and analytics market is segmented into component, deployment model, end user, region. Based on component, the market is classified into software, services and hardware. Based on deployment model, the market is classified into on-premises, cloud-based and hybrid. Based on end user, the market is classified into pharmaceutical and biopharmaceutical companies, contract research organizations (CROs), healthcare providers and regulatory bodies.
Software: The software segment dominated the market in 2024. Software constitutes the nucleus of advanced pharmaceutical statistics and analytics markets and encompasses such things as data assimilation tools, predictive modelling, visualization, and many others. These platforms make it possible for pharmaceutical organizations to manage huge datasets effectively to uncover the latest trends in drugs as they relate to clinical trials or regulatory compliance. Increasing popularity is being observed about advanced analytics software such as AI-powered platforms and machine learning algorithms. They are helping professionals make more informed decisions and streamline R&D pipelines by providing superior scope comfort and customization to meet growing requirements related to data handling.
Services: Services play a crucial role in the new realm of pharmaceutical data and analytics by facilitating the deployment, integration, and maintenance of analytics solutions. Such services would include consultancy, which would be useful in framing the data strategy, and a program to train up the staff along with technical support that is to be given to ensure smooth operations. Increasingly fast traction is being garnered by new services, allowing companies to have data analysis tasks outsourced while they concentrate on what they do more pointedly.
Hardware: It is this hardware that is mainly responsible for powering the necessary computing and storage capacity of pharmaceutical data and analytics systems. That is, the aggregate ensemble of servers, a high-performance computer, and data servers used to process and managing large volumes of data in pharmaceuticals. It is an architectural problem, resulting in everything from big data-or at least cloud-based computation today. A robust bonding hardware solution is very important in getting more fluent integration and reliable performance of analytics software that will provide facilities with more uninterrupted operations.
On-Premises: On-premise deployments require building data analytics solutions locally in an organization's server setup furnished by an organization. It gives more control over the most sensitive of pharmaceutical data and ensures compliance with many security and rigorous regulatory requirements. It tends to be employed by the large pharmaceutical organizations having a sufficient IT resource to manage and maintain the infrastructure. Nonetheless, in terms of on-premise solutions, there are significant initial investments and continued maintenance alongside them, which can make them less suitable for small organizations.
Cloud-Based: Cloud-enabled deployment is now very much preferred in pharmaceutical data and analytics due to its scalability, price, and ease of use. It enables organizations to store and analyze their data with the data located on remote servers, which lines up real-time coordination among people across a company so they can make decisions faster. Another reason that this one is particularly nice is that such solutions, as they keep minimizing the need for setting up larger IT network infrastructure, are generally best-suited for small and medium-sized enterprises (SMEs). Advances in cloud security have allayed apprehensions about data protection and spawned more adoption.
Hybrid: Hybrid deployments join the strengths of on-premise and cloud into a better approach for managing data. Data that is more sensitive may be kept on panels, but cloud platforms can be used for scalability and advanced capabilities of analytics available from the latest models. The flexible nature of this model enables cost optimization even as it upholds security and also compliance while meeting data needs that different kinds of pharmaceutical companies may have due to the varying regulatory environments that they have to navigate.
Pharmaceutical and Biopharmaceutical Companies: The Pharmaceutical and Biopharmaceutical Companies segment dominated the market in 2024. The analysis of data is the main area of application for pharma and biopharma companies that implement these solutions to develop drug discovery, clinical development and manufacturing. Analytics can be leveraged for accelerating R&D, shortening time-to-market and enabling smarter decision making through predictive modelling and real-world evidence. Data analytics is also utilised by such firms in the marketing function, as well as for applications on patient care. With an exponential increase of competition in the industry, the solution based on data plays a crucial role in innovation, regulatory compliance, and efficiency.
Contract Research Organizations (CROs): Contract Research Organizations (CROs) rely to a significant measure on pharma data analytics for the monitoring of clinical trials, patient protection and regulatory compliance. Analytics tools provide the tools for CRC's to conveniently adjust trial designs, increase accrual, and analyze results appropriately. Through the use of advanced analytics, CROs can lower clients' costs both in terms of time and money as well as achieve top quality research results. With increasing outsourcing of R&D functions by the pharma industry, there continues to be a demand for CROs that serve the analytics vendors.
Healthcare Providers: Pharmaceutical data analytics is used by clinical practitioners to improve patient care and to enhance patient outcomes. Based on the analysis of patient file, treatment information and efficacy reports of drugs, clinicians are situated to provide personalized medicine and therefore lead to better treatment decision making. Analytics can track adverse drug reactions and, consequently, can have a preventive role in medication safety. With digital tool use in healthcare systems, providers are now among the key market participants in the field of pharmaceutical analytics and use data-driven insights to further strengthen the pharma-industry collaboration.
Regulatory Bodies: Pharmacovigilance data analytics are used by regulatory agencies to assess the safety, effectiveness, and adherence to the law of pharmaceuticals. In the field of clinical trials, advanced analytics help us guideline the regulator's decision on CT (clinical trial) data, adverse drug reaction monitoring and drug approval transparency. With the help of analytics, these authorities can fine-tune the process of decision-making and better monitor the drug- and medical-device-post-market surveillance. With regulatory environments rapidly data-driven, analytics solutions are crucial for determining how to perform effective and accurate assessments of pharmaceutical products.
The pharmaceutical data and analytics market is segmented into various regions, including North America, Europe, Asia-Pacific, and LAMEA. Here is a brief overview of each region:
The North America region dominated the market in 2024, due to the highly sophisticated nature of healthcare coalitions, wide adoption of digital technologies and significant R&D investments in the pharmaceutical market. Market growth is also behind the trend that there are large scale participants in the market, increased adoption of real-world evidence and regulatory endorsement of data-driven approaches in drug development. In particular, the United States is leading the development of AI-based analytics cloud-based solutions. Continuous high demand for personalized medicine and for efficient clinical trials is driving the application of analysis tools in the area.
European Union (EU) is one of the major markets for pharmaceutical data and analytics, regulatory interest as well as with an emphasis on innovation. There are countries on the globe, including Germany, Great Britain, and Switzerland, who have already started to apply advanced analyses to support drug discovery and clinical trials. In this area, it also benefits from implementation of initiatives to promote the digitisation of healthcare and the use of real-world evidence in regulatory decision-making. The need to reduce the cost of health care delivery and improve patient outcomes has led to the increased use of data analytics across the pharmaceutical and biopharmaceutical industries.
Asia-Pacific market is growing at a fast pace, thanks to the growth in the healthcare digitization, growing pharmaceutical R&D investments and adoption of analytics solutions. Developing countries such as China and India play a significant role, supported by government actions and the increasing number of pharmaceutical manufacturing sites. Supporting the large base of patient populace and high-burden chronic diseases in the area are unique opportunities for data-driven drug development and clinical trials. Due to a rise in demand of cost-effective strategies, clouds-based analytics tools and AI-powered platforms are rapidly expanding.
The LAMEA region is a slowly growing market driven by healthcare transformation and the growing acceptance of data-driven methodologies. In Latin America, both Brazil and Mexico are at present applying analytics to enhance clinical trials as well as regulatory compliance. Public sector health service transformation and productivity spending drive market growth in the Middle East and Africa. Nevertheless, some challenges including IT resource constraints and regulatory difficulties remain. Although, the region represents uncontested potential for analytics companies as healthcare networks evolve.
Market Segmentation
By Component
By Deployment Model
By End User
By Region
Chapter 1. Market Introduction and Overview
1.1 Market Definition and Scope
1.1.1 Overview of Pharmaceutical Data and Analytics
1.1.2 Scope of the Study
1.1.3 Research Timeframe
1.2 Research Methodology and Approach
1.2.1 Methodology Overview
1.2.2 Data Sources and Validation
1.2.3 Key Assumptions and Limitations
Chapter 2. Executive Summary
2.1 Market Highlights and Snapshot
2.2 Key Insights by Segments
2.2.1 By Component Overview
2.2.2 By Deployment Model Overview
2.2.3 By End User Overview
2.3 Competitive Overview
Chapter 3. Global Impact Analysis
3.1 Russia-Ukraine Conflict: Global Market Implications
3.2 Regulatory and Policy Changes Impacting Global Markets
Chapter 4. Market Dynamics and Trends
4.1 Market Dynamics
4.1.1 Market Drivers
4.1.1.1 IoMT Devices
4.1.1.2 Emerging Markets Growth
4.1.2 Market Restraints
4.1.2.1 Data Privacy and Security Concerns
4.1.2.2 High Implementation Costs
4.1.3 Market Challenges
4.1.3.1 Lack of Standardization
4.1.3.2 Resistance to Technology Adoption
4.1.4 Market Opportunities
4.1.4.1 Integrated Analytics Platforms
4.1.4.2 Expansion in Emerging Markets
4.2 Market Trends
Chapter 5. Premium Insights and Analysis
5.1 Global Pharmaceutical Data and Analytics Market Dynamics, Impact Analysis
5.2 Porter’s Five Forces Analysis
5.2.1 Bargaining Power of Suppliers
5.2.2 Bargaining Power of Buyers
5.2.3 Threat of Substitute Products
5.2.4 Rivalry among Existing Firms
5.2.5 Threat of New Entrants
5.3 PESTEL Analysis
5.4 Value Chain Analysis
5.5 Product Pricing Analysis
5.6 Vendor Landscape
5.6.1 List of Buyers
5.6.2 List of Suppliers
Chapter 6. Pharmaceutical Data and Analytics Market, By Component
6.1 Global Pharmaceutical Data and Analytics Market Snapshot, By Component
6.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
6.1.1.1 Software
6.1.1.2 Services
6.1.1.3 Hardware
Chapter 7. Pharmaceutical Data and Analytics Market, By Deployment Model
7.1 Global Pharmaceutical Data and Analytics Market Snapshot, By Deployment Model
7.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
7.1.1.1 On-Premises
7.1.1.2 Cloud-Based
7.1.1.3 Hybrid
Chapter 8. Pharmaceutical Data and Analytics Market, By End-User
8.1 Global Pharmaceutical Data and Analytics Market Snapshot, By End-User
8.1.1 Market Revenue (($Billion) and Growth Rate (%), 2022-2034
8.1.1.1 Pharmaceutical and Biopharmaceutical Companies
8.1.1.2 Contract Research Organizations (CROs)
8.1.1.3 Healthcare Providers
8.1.1.4 Regulatory Bodies
Chapter 9. Pharmaceutical Data and Analytics Market, By Region
9.1 Overview
9.2 Pharmaceutical Data and Analytics Market Revenue Share, By Region 2024 (%)
9.3 Global Pharmaceutical Data and Analytics Market, By Region
9.3.1 Market Size and Forecast
9.4 North America
9.4.1 North America Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.4.2 Market Size and Forecast
9.4.3 North America Pharmaceutical Data and Analytics Market, By Country
9.4.4 U.S.
9.4.4.1 U.S. Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.4.4.2 Market Size and Forecast
9.4.4.3 U.S. Market Segmental Analysis
9.4.5 Canada
9.4.5.1 Canada Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.4.5.2 Market Size and Forecast
9.4.5.3 Canada Market Segmental Analysis
9.4.6 Mexico
9.4.6.1 Mexico Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.4.6.2 Market Size and Forecast
9.4.6.3 Mexico Market Segmental Analysis
9.5 Europe
9.5.1 Europe Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.5.2 Market Size and Forecast
9.5.3 Europe Pharmaceutical Data and Analytics Market, By Country
9.5.4 UK
9.5.4.1 UK Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.5.4.2 Market Size and Forecast
9.5.4.3 UKMarket Segmental Analysis
9.5.5 France
9.5.5.1 France Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.5.5.2 Market Size and Forecast
9.5.5.3 FranceMarket Segmental Analysis
9.5.6 Germany
9.5.6.1 Germany Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.5.6.2 Market Size and Forecast
9.5.6.3 GermanyMarket Segmental Analysis
9.5.7 Rest of Europe
9.5.7.1 Rest of Europe Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.5.7.2 Market Size and Forecast
9.5.7.3 Rest of EuropeMarket Segmental Analysis
9.6 Asia Pacific
9.6.1 Asia Pacific Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.2 Market Size and Forecast
9.6.3 Asia Pacific Pharmaceutical Data and Analytics Market, By Country
9.6.4 China
9.6.4.1 China Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.4.2 Market Size and Forecast
9.6.4.3 ChinaMarket Segmental Analysis
9.6.5 Japan
9.6.5.1 Japan Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.5.2 Market Size and Forecast
9.6.5.3 JapanMarket Segmental Analysis
9.6.6 India
9.6.6.1 India Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.6.2 Market Size and Forecast
9.6.6.3 IndiaMarket Segmental Analysis
9.6.7 Australia
9.6.7.1 Australia Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.7.2 Market Size and Forecast
9.6.7.3 AustraliaMarket Segmental Analysis
9.6.8 Rest of Asia Pacific
9.6.8.1 Rest of Asia Pacific Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.6.8.2 Market Size and Forecast
9.6.8.3 Rest of Asia PacificMarket Segmental Analysis
9.7 LAMEA
9.7.1 LAMEA Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.7.2 Market Size and Forecast
9.7.3 LAMEA Pharmaceutical Data and Analytics Market, By Country
9.7.4 GCC
9.7.4.1 GCC Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.7.4.2 Market Size and Forecast
9.7.4.3 GCCMarket Segmental Analysis
9.7.5 Africa
9.7.5.1 Africa Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.7.5.2 Market Size and Forecast
9.7.5.3 AfricaMarket Segmental Analysis
9.7.6 Brazil
9.7.6.1 Brazil Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.7.6.2 Market Size and Forecast
9.7.6.3 BrazilMarket Segmental Analysis
9.7.7 Rest of LAMEA
9.7.7.1 Rest of LAMEA Pharmaceutical Data and Analytics Market Revenue, 2022-2034 ($Billion)
9.7.7.2 Market Size and Forecast
9.7.7.3 Rest of LAMEAMarket Segmental Analysis
Chapter 10. Competitive Landscape
10.1 Competitor Strategic Analysis
10.1.1 Top Player Positioning/Market Share Analysis
10.1.2 Top Winning Strategies, By Company, 2022-2024
10.1.3 Competitive Analysis By Revenue, 2022-2024
10.2 Recent Developments by the Market Contributors (2024)
Chapter 11. Company Profiles
11.1 TRINITY PHARMA SOLUTIONS
11.1.1 Company Snapshot
11.1.2 Company and Business Overview
11.1.3 Financial KPIs
11.1.4 Product/Service Portfolio
11.1.5 Strategic Growth
11.1.6 Global Footprints
11.1.7 Recent Development
11.1.8 SWOT Analysis
11.2 CitiusTech Inc.
11.3 IBM
11.4 International Business Machines Corporation
11.5 Northwest Analytics Inc.
11.6 ORACLE
11.7 SCIO HEALTH ANALYTICS
11.8 Statistical Analysis System
11.9 TAKE Solutions Ltd.
11.10 Wipro