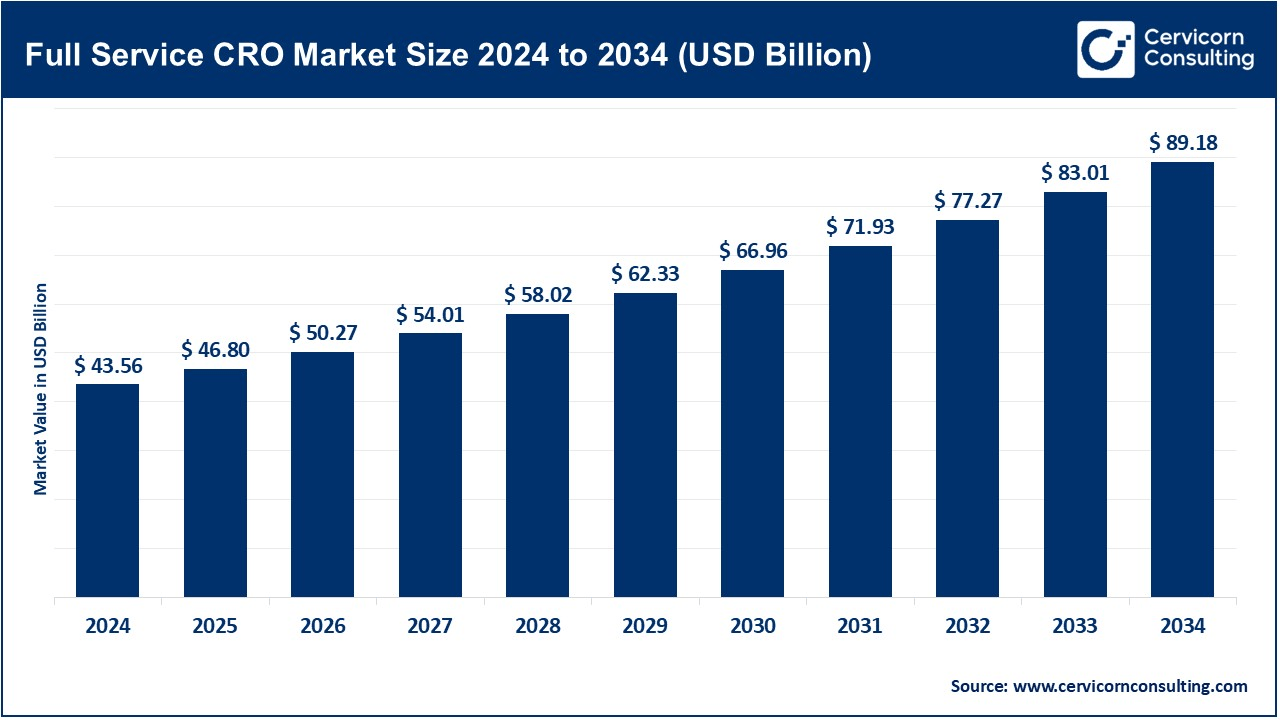
The global full service CRO market size was valued at USD 43.56 billion in 2024 and is expected to reach around USD 89.18 billion by 2034, growing at a compound annual growth rate (CAGR) of 7.42% over the forecast period from 2025 to 2034. The full service CRO market is growing fast with a rising demand for outsourced clinical research services, especially by pharmaceutical and biotech companies that wish to focus on core activities by outsourcing non-core tasks. Companies find it prudent to partner with full service CROs for the increasing complexity of clinical trials along with regulatory challenges and the need for specialized expertise. Growing demand for novel drugs and therapies, along with rising focus on clinical trial efficiency and cost control, are likely to drive growth for the market. This is because outsourcing at various phases of clinical trials remains a compelling factor driving this market.
A full-service Contract Research Organization provides expansive clinical research services for organizations involved in the pharmaceutical, biotechnology, and medical device industries. A full-service CRO handles every phase of clinical trials-from design and planning to execution, data management, regulation submissions, and post-market activities. These organizations also provide specialized knowledge and skills in clinical trial management, site monitoring, patient recruitment, and quality assurance, which enables pharmaceutical companies to outsource all of their clinical trial needs. Full-service CROs assist clients in navigating the complex regulatory environment and speeding development and approval of new drugs and therapies.
Report Scope
| Area of Focus | Details |
| Market Size in 2025 | USD 46.80 Billion |
| Expected Market Size in 2034 | USD 89.18 Billion |
| Projected CAGR 2025 to 2034 | 7.42% |
| Dominant Region | North America |
| Growing Region | Asia-Pacific |
| Key Segments | Type, Application, End User, Region |
| Key Companies | Medpace, Laboratory Corporation of America Holdings, ICON plc, IQVIA Inc, Syneos Health, Novotech, KCR S.A., PSI, Ergomed Group, Thermo Fisher Scientific Inc., WuXi Biologics, Tigermed, Worldwide Clinical Trials, Charles River Laboratories, Microbiologic, Parexel International (MA) Corporation |
Early Phase Development Services: Early-phase development services comprise preclinical studies, phase one clinical studies and proof-of-concept studies of a full-service market CRO that determine the safe dosage and biologic activity for a new therapeutic or drug product. The other support offered through CRO's includes study designing, regulatory submittals along with early-time management of first-in-human phase clinical trials reducing risks and allowing pharmaceutical companies an accelerated time to market for developing new drugs.
Clinical: The full-service CRO provides a range of services, from phase II to phase IV clinical trials that are concerned with the assessment of efficacy, safety, and the long-term drug or medical devices. Such services include recruitment, monitoring of patients, collection of data, and regulatory support. Full-service CROs will collaborate with the sponsors in relation to the design and management of a clinical trial with post-market surveillance, observing the set-up of regulatory compliance and gaining worthwhile information for new therapies approval and commercialization.
Laboratory Service: Laboratory services include full-service kinds of laboratory testing and analysis that support the conduct of clinical trials, such as testing for biomarkers, pharmacokinetic studies, and diagnostic assays. Validity of data collected in these clinical trials gets allowed through its services. Often, these services offered by full-service CROs may include: specialized testing as genetic sequencing; drug metabolism studies as well as analysis of tissue necessary for the burgeoning growth of the personalized medicine market and biologics.
Oncology: Oncology is one of the largest segments in the full-service CRO market due to the highly complex nature of cancer therapies, in addition to the more massive level of expertise needed to manage clinical trials involving this disease. Services for oncology trials, such as patient recruitment, molecular testing, and regulatory compliance, are offered by CROs. The more ongoing development of new cancer drugs and immunotherapies creates the demand for CROs to have the ability to handle these specific clinical trials for the progression of successful cancer therapies.
Neurology: Neurology applications in the CRO market are focused on clinical trials on neurological disorders such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, and epilepsy. These diseases typically involve complex trial designs because they present differently in symptoms and have a long disease progression course. Full-service CROs provide specialized services in neuroimaging, cognitive testing, and patient monitoring to spur the development of new therapies. Thus, this application is an emerging trend in the CRO market.
Cardiology: There is a development of treatments for those diagnosed with a disease, whether it is heart disease, hypertension, arrhythmias, and other cardiovascular conditions. Full-service CROs are important for their services are basic to be conducted in all cardiac monitoring and biomarker tests and recruitment for cardiology studies. With cardiovascular disorders remaining one of the biggest killers worldwide, an interest in novel treatments is seen and thus requires a CRO that has experience managing cardiology-based clinical study portfolios.
Infectious disease: Infectious disease applications range from clinical research in HIV/AIDS, tuberculosis, and malaria to COVID-19 very recently. In this segment, full-service CROs help with vaccine, antiviral drugs, and diagnostic tools development. The rapid global spread of infectious diseases underscores the urgency of completing clinical trials expeditiously in the development of treatments and vaccines. CROs play a vital role in securing expedited regulatory approvals and efficient designs of trials for such diseases.
Metabolic Disorder: Metabolic disorders include diabetes and obesity along with other endocrine-related diseases. In most clinical trials pertaining to this area, patients are being monitored for a long-term period and adherence among them needs to be maintained towards the treatment plan. Full-service CROs provide support in this direction by ensuring proper recruitment of patients, data collection, and adherence to regulatory requirements. As the incidence of metabolic disorders remains on the upsurge all over the world, it becomes of utmost importance to speed up development of treatments/therapies for such chronic diseases in collaboration with CROs.
Renal/Nephrology: Renal or nephrology applications in the CRO market are concentrated on clinical trials for kidney diseases such as chronic kidney disease, dialysis, and kidney transplants. In such cases, the services that a CRO provides include recruitment of patients, monitoring of renal functions, and other laboratory tests specialized to the study. Since kidney diseases are highly prevalent, especially in the aging population, CROs are playing an important role in supporting the development of new therapies, including new treatments for renal failure and drugs to retard the disease's progression.
Pharmaceutical & Biotechnology Companies: Major end-users of full-service CROs are pharmaceutical and biotechnology companies. Companies have to outsource third-party management over clinical trials, regulatory submissions, and data analysis. It hires the company to help stream line their activities, reduce cost, and get new drugs to the market faster. Full-service CROs are helping pharmaceutical and biotech firms bring drugs and therapies from early-phase research to post-market surveillance.
Medical Device Companies: This other area of full-service CRO applications comes by the medical device manufacturing companies that handle clinical trials conducted to have their products approved from the regulatory body.The medical device trials entail safety and effectiveness of the product, besides maintaining strict health and safety standards. CROs help in all types of trials, for instance, device testing know-how, data management, patient observation, and reporting. The need for CROs is a direct result of growth that has been experienced in the medical device industry.
Academic & Research Institutes: Academic and research organizations have often collaborated with full-service CROs as a means of accessing the required expertise, infrastructures, and technologies needed to run clinical trials. Through such partnerships, academic institutes can accelerate their research by leveraging the services provided by CROs, including clinical trial management, regulatory guidance, and patient recruitment. CROs help different areas of academic research, including early-phase studies, proof-of-concept trials, and regulatory affairs, to translate basic research into clinical practice.
The full service CRO market is segmented into several key regions: North America, Europe, Asia-Pacific, and LAMEA (Latin America, Middle East, and Africa). Here’s an in-depth look at each region.
North America remains the largest and most mature market for full-service contract research organizations (CROs). These are primarily fueled by pharmaceutical, biotechnology, and medical device companies. Specifically, the United States dominates with its huge infrastructure of healthcare and a massive population, and hence, the enormous investment in research and development activities. The principal cities in which clinical trials take place and new drugs are being developed are New York, San Francisco, and Boston. Canada is another very important market with growing interest in clinical research, a favorable regulatory environment, and partnerships with worldwide CROs. For instance, Verdiva announced raising USD 410 million in Series A funding in January 2025 to further drive the development of weekly-dosed weight loss medicines. The money would go to late-stage clinical trials and further expanding company pipelines in new products. The growth of Verdiva will be fueled by the funding with a very aggressive weight management market focused on treating obesity and all its related conditions.
Europe is another main region for the full-service CRO market. Countries such as Germany, the United Kingdom, France, and Switzerland are on the top lists in clinical research and development. A large, integrated market for CROs in the European Union (EU) is provided due to its harmonized regulatory environment and collaborative research efforts across the member states. Both of these countries have advanced health care, and there is also an important biotechnology industry in the UK, which makes these central to CRO activities of the region. There are recent reports saying that in December 2024, the Oxford University researchers work on the very first ovarian cancer prevention vaccine, called OvarianVax. This aims to teach the immune system the detection of cancerous cells from an early age. For three years, researchers will receive funding amounting to USD 614,418 to administer this vaccine in those individuals bearing BRCA1/2 mutation, with an increased chance of ovarian cancer development. Such a vaccine will reduce cancer cases and enhance the survival rate among patients.
The Asia-Pacific region is growing at a fast pace, especially due to expanding pharmaceutical and biotechnology sectors, increased investments in healthcare, and higher patient populations. Countries in which such growth is occurring include China, India, Japan, and South Korea. Clinical trials are highly attractive in China and India because of their size and diversity in population, and their relatively low operating costs. Japan and South Korea are countries known for their rigorous regulatory standards, world-class medical infrastructure, and leading-edge research in areas of oncology and neurology. APAC also receives advantages due to the increasing emphasis on precision medicine and, therefore, is increasingly critical for clinical trial outsourcing.
The region of LAMEA includes Latin America, Middle East, and Africa, the three regions emerging as a hotbed of humongous promise for full service CRO. Brazil, Mexico, and Argentina are drawing the attention of CROs since these countries are characterized by significant patient populations and low costs associated with trials while healthcare infrastructure in these regions is increasingly emphasized. Clinical research today has picked up at all-time high levels in the Middle East, concentrating on United Arab Emirates, Saudi Arabia, and Israel due to advanced medical care, increasing government health investments into research in healthcare sectors, and surging pharmaceutical and biotech industries. Today, in Africa, more clinical researches are underway mainly in the regions of South Africa and Kenya where healthcare services and supports given to the healthcare research activities bring wings to the CRO. However, the region is often marred by regulatory challenges, political instability, and varying healthcare standards across countries.
CEO Statements
August Troendle, CEO of Medpace
"The Full-Service CRO market is poised for significant growth as demand for efficient, personalized healthcare solutions rises. At Medpace, we focus on operational excellence, global reach, and patient-centered approaches to streamline clinical development, reduce costs, and accelerate the delivery of life-changing therapies."
Steve Cutler, CEO of ICON plc
"The Full-Service CRO market is crucial in accelerating the development of new treatments. At ICON, we leverage global expertise, advanced technologies, and therapeutic knowledge to deliver efficient, high-quality solutions, bringing therapies to patients faster and more cost-effectively."
John Moller, CEO of Novotech
"As the Full-Service CRO market grows, the demand for faster and more efficient clinical trials continues to rise. At Novotech, we combine advanced technology with deep local expertise to support our clients in conducting complex global trials. Our mission is to streamline the development of ground breaking therapies and ensure they reach patients more quickly and effectively."
Market Segmentation
By Type
By Application
By End-user
By Region